| Electrons in Atoms: An Atomic Amusement Park Ride Analogy |  |
Consider an analogy between an atom and an amusement park ride. The idea is to think of electrons in an atom as the people amusing themselves on the ride. This particular ride (atom) has seats in which one or two people (electrons) can sit. The ride is rather interesting because different seats do different things and at different speeds! The ride has different levels (shells) of seating, some of which are closer and some farther from the center of the ride (the nucleus).
Level 1: The set closest to the center only has one two-electron seat. This seat goes in and out from the nucleus in different directions. This seat is for the people who don't want to spend much on the ride or for those who don't like to whirl around on rides.
Level 2: The second set of seats costs a bit more, but the ride is a bit more interesting. There are four two-electron seats in this set: one seat that goes in and out as before, but it goes farther out and travels faster at times, and three seats that go around the center, each at a different orientation or angle, sort of like three merry-go-rounds or Ferris wheels.
Level 3: The seats in the third set are even more expensive and there are nine two-electron seats. There is one two-electron seat that just goes in and out from the center but farther and faster than level two, three seats that both go around the center and go in and out, and five seats that really whiz around the center all in different ways.
Level 4: There are 16 seats at this level. There is the seat that goes in and out, but really fast and far now. There are three that go around a bit, but go in and out a lot. There are five that go around a lot, and in and out a bit. And there are seven that whiz extremely rapidly about the center, all in different directions.
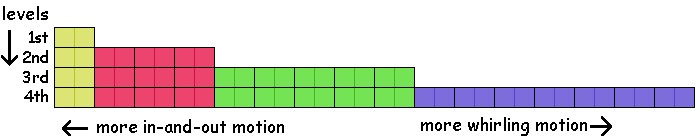
The pattern keeps going on. Level 5 has 25 seats grouped into sets of 1, 3, 5, 7, and 9. (Notice the pattern in the number of seats?) Level 6 has 36 seats and so on. The illustration below shows a "seating chart" for the first four levels. Each seat has spots for two electrons. The first level has one seat (yellow). The second level has one yellow and three red seats. The third level has one yellow, three red, and five green. The fourth has one yellow, three red, five green, and seven blue. The fifth level would have another seat of nine seats, and so on. The more toward the left on the chart, the more the seats move in and out. The more toward the right, the more they whirl around the center.
Let's look more closely at specific elements. Consider hydrogen which only has one electron. On the amusement park ride analogous to a hydrogen atom, the electron has its "choice" of any of those seats. If we had some hydrogen atoms at room temperature, somehow not combined with other atoms, we would find the electron in the first level seat, happily going in and out. In the flame of a candle, in a "neon" light tube filled with hydrogen rather than neon, or in the sun, you might find quite a few atoms of hydrogen where the electron is in one of the various upper level seats, with more energy.
In a typical helium atom at room temperature, its two electrons would both fill the seat on the level. In a lithium atom, two of the three electrons would be in the first level seat and the other would be in a second level seat. With all these electrons in the atom, there is a definite order of filling seats within a level. It turns out that the seats that go in and out get "filled" before the ones that whirl around. The second level seat that goes in and out gets filled first. In a beryllium atom, with four electrons, two are in the first level in-and-out seats and two are in the second level in-and-out seats.
Before we keep going too far, is there a way to track of all this? Yes, The periodic table does it nicely for us. See the next section for details!